This is a guest post by Peter Gill, a retired physicist who may be familiar to readers as a central character in my Institutional Bias pamphlet. I have lightly edited a couple of paragraphs to make the meaning clear.
The ClimateSceptics Yahoo Email Group is very active and includes contributions from some, notably Mike MacCracken, who are certainly not sceptics of the AGW set of hypotheses. On 7 January 2015, Mike MacCracken wrote two pieces on carbon dioxide. The first on 7 January included this:
But what I really want to write you about is the statement: “(Also recall than man's CO2 is different than nature's CO2 because man's CO2 magically stays in the atmosphere while nature's doesn't.)” The statement results from a misunderstanding all CO2 molecules are the same and experience the same processes. There are two different times to be aware of:
(a) one is the time for a particular molecule (any molecule) to go from the atmosphere into the upper ocean or to go from the atmosphere to the biosphere and back. So it is the average atmospheric lifetime of a particular molecule. Estimates of fluxes and studies of how fast nuclear test generated carbon-14 give this average residence time for a particular CO2 molecule as of order 4-5 years. These flux exchanges are much larger than the human emissions, leading to suggestions the small human emissions compared to the exchange fluxes can’t have an effect but, as explained in the next point, what matters for climate is the persistence of the CO2 perturbation, not of the lifetime of the particular CO2 molecule.
(b) were the transfer of a particular molecule to the ocean or biosphere all that happened, then the human induced CO2 emissions would not be a problem as the atmospheric perturbation created by human emissions would be pulled back down over a few years. However, when a CO2 molecule goes into the ocean, another one goes from the ocean into the atmosphere (that is what being at equilibrium chemically means) and similarly for a CO2 molecule going into the biosphere (or we’d see a steady growth in the total CO2 content in the biosphere so in trees, etc.). As a result, were the actual set of exchanges perfectly in balance, the increase in the atmospheric concentration of CO2 caused by human activities would stay exactly the same. Now, actually, a bit more goes into the oceans than comes out because a bit of the increased CO2 is transferred to the deep ocean (the down going water has a bit higher CO2 concentration due to its recent contact with the atmosphere than the upward moving water that has a lower CO2 concentration from having been in contact with the atmosphere centuries ago). So, the lifetime of the human-induced perturbation ends up being quite long first the human-induced perturbation is reduced as the CO2 spreads through the deep ocean, but with the added CO2 in the ocean (even though spread out) the human-induced perturbation does not go to zero until the added CO2 is taken up by organisms and deposited on the ocean floor as sediments and that takes many, many millennia. So, the time constant of the perturbation (or actually there are time constants for each of the transfers upper ocean to deep ocean, deep ocean to sediments, etc. is in effect very long. Note that this time constant is for the perturbation, not for a particular molecule as indicated above.I will agree there has been confusion on terminology on all of this, but when understood, it is clear that all CO2 molecules are affected in the same way and the difference is between the time constant for a particular molecule and the time constant for the duration of the perturbation to the overall CO2 concentration. The former is 5 years, and the latter is many, many millennia.
On 8 January Mike added:
And on the CO2 increase being due to humans, that is also an area with lots of various tests and analyses done—well documented and another area that you should look intensively in. [The reason that] IPCC does not cover it now is that this was well-established well before IPCC, which itself focuses on advances in the science.
Finally, in suggesting all sorts of remote possibilities with little or no evidence, you fall into the same, but opposite category of those who suggest the very worst that you and others so roundly criticize. The IPCC and science are seeking the most defensible, most likely, objective and quantifiable explanations. Sure, a comet or asteroid could destroy the Earth next year and so no need to worry about anything—I can’t prove that won’t happen—but we are dealing with the most probable explanations.
On 8 January following the comments in the second of Mike’s contributions I wrote:
I have bit my lip and not entered into this string until now. However given your statement about there being no argument that the increase in CO2 is due to human activity I feel that I have to say a few things.
The so-called proof that the increase in CO2 is anthropogenic is faulted in a number of ways. So from your point of view it is a good job that IPPC has chosen not to repeat the argument. If you would like to detail the isotope argument for the benefit of those who have not heard it I will explain the error in logic.
Mike replied on the same day with:
Okay, so the reasons (very generally stated and as I understand them) making clear the human rather than natural influence:
- Independent compilations of emissions from fossil fuels and biomass destruction/soil oxidation that, along with understanding of the global carbon cycle, match in amount and time history the changes in the atmospheric CO2 concentration (from ice cores before the setting up of the Mauna Loa and other observatories) happening in the atmosphere (except for a perplexing time during late 1930s and early World War II)
- Dilution of the natural C-14 content of the atmospheric CO2 (the natural C-14 being created by cosmic rays) in that fossil fuels have been sequestered much, much longer than the lifetime of C-14, so they are essentially coming in with zero C-14. Yes, there can be fluctuations in C-14 generation, but the dilution effect, integrated over time, is the dominant influence.
- Shifts in the C13/C14 ratio that give good indications of the fractionation between fossil fuel emissions and biomass—noting that care does have to be taken regarding distinguishing C3/C4 crops and their relative roles.
I think that is about it—amount and timing really also match (and per Occam’s Razor, postulating that unique natural processes started and followed with the exact same timing as has occurred for the fossil fuel/agriculture contributions, is pressing improbabilities quite far.
Arthur Rörsch entered the conversation with the following to me:
The ‘proof that’ 50% of anthropogenic emissions has been accumulating is based on the solution of one equation with two unknowns and the neglect of the grand CO2 cycle. It is assumed that approximately 150 GtC/y is brought from the deep sea into the atmosphere (F in) in the equatorial regions and also approximately the same amount (F out) is returned to the deep sea near the polar regions. The residence time of CO2 in the deep sea is of the order of magnitude of 1000-2000 years. The two variables over decades are uncoupled and we do not have figures based on observations.
We can only measure the accumulation dA/dt and make a rough estimate of the human emission rate dE/dt = 10 GtC/y
The equation reads : dA/dt = F in + dE/dt - F out
With two unknowns and their variability F in and F out.
The idea that dA/dt = 0.5 dE/dt follows from this equation, without knowing the natural variability of F in and F out. It is one of the examples of easily jumping to conclusions without recognising the effect of natural variability.
The isotope measurements C12/C13 show clearly that CO2 from burning of fossil fuels has been taken up in the cycle. They also show the downward flux to the deep sea near the poles. The C12/C13 label is however rather weak and there are some discrepancies in the measurements. But it is helpful as a scientific instrument to study the major flows in and out of the oceans. But as usual in climate research it is not easy to understand what is cause and what is effect.
My holding reply was:
Thanks to both Arthur R and Mick M. I hope to respond over this coming weekend. It is an important matter as whilst the overall AGW hypothesis consists of around half a dozen interacting hypotheses the origin of the increase in CO2 in recent centuries is crucial to attribution.
Those like myself operating gmail account will no doubt be frustrated by the way in which Google piles e-mails on top of each other making following a string rather difficult especially with multi-contributors who are following different aspects of the matter under discussion or even raising new topics. So, although the following response will be included in the Climate Sceptics string, I shall also provide it as a guest post on Bishop Hill http://bishophill.squarespace.com/
In what follows I do not address the question of the importance or otherwise of carbon dioxide in heat-loss calculations. The post only concerns the origin of the increase in atmospheric carbon dioxide over the last couple of hundred years.
Some of those who support the AGW hypotheses believe crudely that all the carbon dioxide from fossil fuel burning from the beginning of the Industrial Revolution effectively remains in the atmosphere. David MacKay is one such believer. Others, including Mike, have a different take on the topic as indicated above but still maintain that the reason for the increase in carbon dioxide levels is the burning of fossil fuels by mankind.
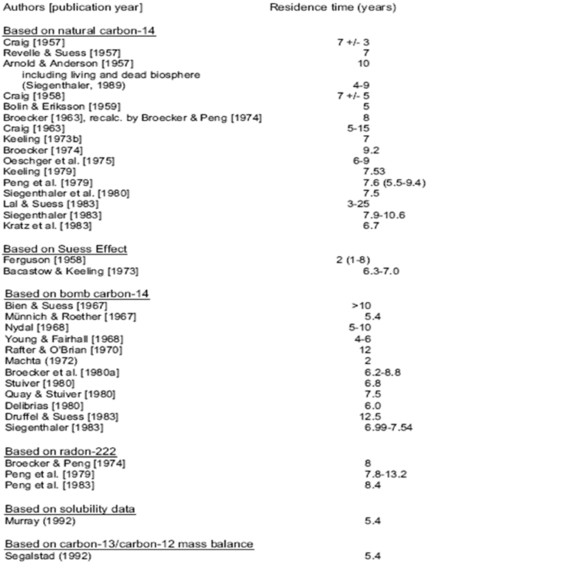
Mike gave a little background to the very different time constants relating to carbon dioxide residence times. This requires a little more amplification. In fact in the period before the advent of IPCC there were over 30 different determinations of the effective lifetime of CO2 in the atmosphere as follows.
The effective residence time range from all these determinations is 4—25 years, with almost all studies concluding that the maximum residence time was less than 15 years and the majority of studies indicating under 10 years. One study concluded a residence time of circa 25 years. Nevertheless, Mike is right to conclude that the residence time is circa 5 years on average. He gives a range of 4—5 years whereas the whole range of studies gives 5—6 years. Of course, this contrasts somewhat with IPPC’s assumption for models of circa 100 years but without going into the matter in more detail this is probably because IPCC assume that the natural level to which CO2 needs to fall is its stated and so-called pre-industrial level of 280 ppm.
There is another conclusion that can be drawn from the data, which is that the behaviour of both CO2 sources and sinks are variable. On this basis it may be further concluded that the variability could easily exceed the amount of current annual anthropogenic CO2 by a factor of ten thus leaving anthropogenic emissions in the noise.
I will move now to the major issue: the so-called anthropogenic fingerprint. It is known that plants have a slight preference for the C12 isotope of carbon and animals making shells have a slight preference for C13. C14, made from nitrogen by bombardment with cosmic rays, is a useful dating tool. As coal is composed of fossilised plants it is argued that by burning it and thereby increasing the C12/C13 ratio the latter is an indication that the source of the increase of carbon dioxide in the atmosphere is anthropogenic. However let us suppose, as the major fluxes are natural, that in fact the recent (circa 200 years) increase in CO2 is also natural. In that case the proportion from sea and soil out-gassing is to some extent governed by Henry’s Law. Simply stated “The quantity of a gas dissolved in a liquid at a particular temperature is proportional to the pressure of that gas above the liquid”. So if one burns coal then the contribution of the carbon dioxide combustion product contributes to the partial pressure of atmospheric CO2 and depresses an equal amount of what would otherwise have been out-gassed from the sea. Of course this still means that the C12/C13 ratio is affected and the wrong conclusion reached by anyone not appreciating the subtlety of the partial pressure effect.
There are other arguments for and against either proposition concerning the increase in atmospheric CO2 but for simplicity here and to allow for reader comment these have not been ventilated here.
By the way, even the IPCC's assumption of the pre-industrial amount of CO2 in the atmosphere, which in turn relies on the interpretation of ice cores (Siple for example), can be challenged and hence the knock-on effects in terms of long-term residence times can be challenged too, but that’s another story.