 Bishop Hill
Bishop Hill Carbon conversation
 Jan 15, 2015
Jan 15, 2015  Climate: carbon budget
Climate: carbon budget This is a guest post by Peter Gill, a retired physicist who may be familiar to readers as a central character in my Institutional Bias pamphlet. I have lightly edited a couple of paragraphs to make the meaning clear.
The ClimateSceptics Yahoo Email Group is very active and includes contributions from some, notably Mike MacCracken, who are certainly not sceptics of the AGW set of hypotheses. On 7 January 2015, Mike MacCracken wrote two pieces on carbon dioxide. The first on 7 January included this:
But what I really want to write you about is the statement: “(Also recall than man's CO2 is different than nature's CO2 because man's CO2 magically stays in the atmosphere while nature's doesn't.)” The statement results from a misunderstanding all CO2 molecules are the same and experience the same processes. There are two different times to be aware of:
(a) one is the time for a particular molecule (any molecule) to go from the atmosphere into the upper ocean or to go from the atmosphere to the biosphere and back. So it is the average atmospheric lifetime of a particular molecule. Estimates of fluxes and studies of how fast nuclear test generated carbon-14 give this average residence time for a particular CO2 molecule as of order 4-5 years. These flux exchanges are much larger than the human emissions, leading to suggestions the small human emissions compared to the exchange fluxes can’t have an effect but, as explained in the next point, what matters for climate is the persistence of the CO2 perturbation, not of the lifetime of the particular CO2 molecule.
(b) were the transfer of a particular molecule to the ocean or biosphere all that happened, then the human induced CO2 emissions would not be a problem as the atmospheric perturbation created by human emissions would be pulled back down over a few years. However, when a CO2 molecule goes into the ocean, another one goes from the ocean into the atmosphere (that is what being at equilibrium chemically means) and similarly for a CO2 molecule going into the biosphere (or we’d see a steady growth in the total CO2 content in the biosphere so in trees, etc.). As a result, were the actual set of exchanges perfectly in balance, the increase in the atmospheric concentration of CO2 caused by human activities would stay exactly the same. Now, actually, a bit more goes into the oceans than comes out because a bit of the increased CO2 is transferred to the deep ocean (the down going water has a bit higher CO2 concentration due to its recent contact with the atmosphere than the upward moving water that has a lower CO2 concentration from having been in contact with the atmosphere centuries ago). So, the lifetime of the human-induced perturbation ends up being quite long first the human-induced perturbation is reduced as the CO2 spreads through the deep ocean, but with the added CO2 in the ocean (even though spread out) the human-induced perturbation does not go to zero until the added CO2 is taken up by organisms and deposited on the ocean floor as sediments and that takes many, many millennia. So, the time constant of the perturbation (or actually there are time constants for each of the transfers upper ocean to deep ocean, deep ocean to sediments, etc. is in effect very long. Note that this time constant is for the perturbation, not for a particular molecule as indicated above.I will agree there has been confusion on terminology on all of this, but when understood, it is clear that all CO2 molecules are affected in the same way and the difference is between the time constant for a particular molecule and the time constant for the duration of the perturbation to the overall CO2 concentration. The former is 5 years, and the latter is many, many millennia.
On 8 January Mike added:
And on the CO2 increase being due to humans, that is also an area with lots of various tests and analyses done—well documented and another area that you should look intensively in. [The reason that] IPCC does not cover it now is that this was well-established well before IPCC, which itself focuses on advances in the science.
Finally, in suggesting all sorts of remote possibilities with little or no evidence, you fall into the same, but opposite category of those who suggest the very worst that you and others so roundly criticize. The IPCC and science are seeking the most defensible, most likely, objective and quantifiable explanations. Sure, a comet or asteroid could destroy the Earth next year and so no need to worry about anything—I can’t prove that won’t happen—but we are dealing with the most probable explanations.
On 8 January following the comments in the second of Mike’s contributions I wrote:
I have bit my lip and not entered into this string until now. However given your statement about there being no argument that the increase in CO2 is due to human activity I feel that I have to say a few things.
The so-called proof that the increase in CO2 is anthropogenic is faulted in a number of ways. So from your point of view it is a good job that IPPC has chosen not to repeat the argument. If you would like to detail the isotope argument for the benefit of those who have not heard it I will explain the error in logic.
Mike replied on the same day with:
Okay, so the reasons (very generally stated and as I understand them) making clear the human rather than natural influence:
- Independent compilations of emissions from fossil fuels and biomass destruction/soil oxidation that, along with understanding of the global carbon cycle, match in amount and time history the changes in the atmospheric CO2 concentration (from ice cores before the setting up of the Mauna Loa and other observatories) happening in the atmosphere (except for a perplexing time during late 1930s and early World War II)
- Dilution of the natural C-14 content of the atmospheric CO2 (the natural C-14 being created by cosmic rays) in that fossil fuels have been sequestered much, much longer than the lifetime of C-14, so they are essentially coming in with zero C-14. Yes, there can be fluctuations in C-14 generation, but the dilution effect, integrated over time, is the dominant influence.
- Shifts in the C13/C14 ratio that give good indications of the fractionation between fossil fuel emissions and biomass—noting that care does have to be taken regarding distinguishing C3/C4 crops and their relative roles.
I think that is about it—amount and timing really also match (and per Occam’s Razor, postulating that unique natural processes started and followed with the exact same timing as has occurred for the fossil fuel/agriculture contributions, is pressing improbabilities quite far.
Arthur Rörsch entered the conversation with the following to me:
The ‘proof that’ 50% of anthropogenic emissions has been accumulating is based on the solution of one equation with two unknowns and the neglect of the grand CO2 cycle. It is assumed that approximately 150 GtC/y is brought from the deep sea into the atmosphere (F in) in the equatorial regions and also approximately the same amount (F out) is returned to the deep sea near the polar regions. The residence time of CO2 in the deep sea is of the order of magnitude of 1000-2000 years. The two variables over decades are uncoupled and we do not have figures based on observations.
We can only measure the accumulation dA/dt and make a rough estimate of the human emission rate dE/dt = 10 GtC/y
The equation reads : dA/dt = F in + dE/dt - F out
With two unknowns and their variability F in and F out.
The idea that dA/dt = 0.5 dE/dt follows from this equation, without knowing the natural variability of F in and F out. It is one of the examples of easily jumping to conclusions without recognising the effect of natural variability.
The isotope measurements C12/C13 show clearly that CO2 from burning of fossil fuels has been taken up in the cycle. They also show the downward flux to the deep sea near the poles. The C12/C13 label is however rather weak and there are some discrepancies in the measurements. But it is helpful as a scientific instrument to study the major flows in and out of the oceans. But as usual in climate research it is not easy to understand what is cause and what is effect.
My holding reply was:
Thanks to both Arthur R and Mick M. I hope to respond over this coming weekend. It is an important matter as whilst the overall AGW hypothesis consists of around half a dozen interacting hypotheses the origin of the increase in CO2 in recent centuries is crucial to attribution.
Those like myself operating gmail account will no doubt be frustrated by the way in which Google piles e-mails on top of each other making following a string rather difficult especially with multi-contributors who are following different aspects of the matter under discussion or even raising new topics. So, although the following response will be included in the Climate Sceptics string, I shall also provide it as a guest post on Bishop Hill http://bishophill.squarespace.com/
In what follows I do not address the question of the importance or otherwise of carbon dioxide in heat-loss calculations. The post only concerns the origin of the increase in atmospheric carbon dioxide over the last couple of hundred years.
Some of those who support the AGW hypotheses believe crudely that all the carbon dioxide from fossil fuel burning from the beginning of the Industrial Revolution effectively remains in the atmosphere. David MacKay is one such believer. Others, including Mike, have a different take on the topic as indicated above but still maintain that the reason for the increase in carbon dioxide levels is the burning of fossil fuels by mankind.
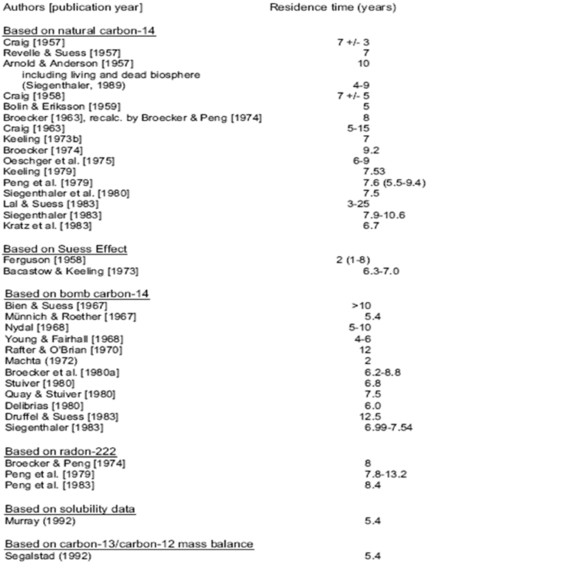
Mike gave a little background to the very different time constants relating to carbon dioxide residence times. This requires a little more amplification. In fact in the period before the advent of IPCC there were over 30 different determinations of the effective lifetime of CO2 in the atmosphere as follows.
The effective residence time range from all these determinations is 4—25 years, with almost all studies concluding that the maximum residence time was less than 15 years and the majority of studies indicating under 10 years. One study concluded a residence time of circa 25 years. Nevertheless, Mike is right to conclude that the residence time is circa 5 years on average. He gives a range of 4—5 years whereas the whole range of studies gives 5—6 years. Of course, this contrasts somewhat with IPPC’s assumption for models of circa 100 years but without going into the matter in more detail this is probably because IPCC assume that the natural level to which CO2 needs to fall is its stated and so-called pre-industrial level of 280 ppm.
There is another conclusion that can be drawn from the data, which is that the behaviour of both CO2 sources and sinks are variable. On this basis it may be further concluded that the variability could easily exceed the amount of current annual anthropogenic CO2 by a factor of ten thus leaving anthropogenic emissions in the noise.
I will move now to the major issue: the so-called anthropogenic fingerprint. It is known that plants have a slight preference for the C12 isotope of carbon and animals making shells have a slight preference for C13. C14, made from nitrogen by bombardment with cosmic rays, is a useful dating tool. As coal is composed of fossilised plants it is argued that by burning it and thereby increasing the C12/C13 ratio the latter is an indication that the source of the increase of carbon dioxide in the atmosphere is anthropogenic. However let us suppose, as the major fluxes are natural, that in fact the recent (circa 200 years) increase in CO2 is also natural. In that case the proportion from sea and soil out-gassing is to some extent governed by Henry’s Law. Simply stated “The quantity of a gas dissolved in a liquid at a particular temperature is proportional to the pressure of that gas above the liquid”. So if one burns coal then the contribution of the carbon dioxide combustion product contributes to the partial pressure of atmospheric CO2 and depresses an equal amount of what would otherwise have been out-gassed from the sea. Of course this still means that the C12/C13 ratio is affected and the wrong conclusion reached by anyone not appreciating the subtlety of the partial pressure effect.
There are other arguments for and against either proposition concerning the increase in atmospheric CO2 but for simplicity here and to allow for reader comment these have not been ventilated here.
By the way, even the IPCC's assumption of the pre-industrial amount of CO2 in the atmosphere, which in turn relies on the interpretation of ice cores (Siple for example), can be challenged and hence the knock-on effects in terms of long-term residence times can be challenged too, but that’s another story.


Reader Comments (43)
Is it generally agreed that the efficacy of sources and sinks varies over time, and in particular with temperature?
An interesting read and pleased to learn a bit more regarding C12 / C 13, Thank you!
Going off on a tangent- as noted, there is a 'slight preference' for the very isotopes of C which we are putting out, one for plants, another for (at least some types of) mollusks. I find that interesting at least with the mollusks, where they keep coming out with research claiming mollusks would have a harder time making shells as CO2 went up. This 'slight preference' seems to me would be taking things in the opposite direction (and that seems to be borne out by evidence in the field).
Everything that affects co² absorbtion and emission changes for sure but the efficiency with which it changes? I have no idea. Effectiveness absolutely.
I am completely ignorant of these matters but my gut feeling is that we do not know very much about the natural sources of CO2 and the quantities involved. Emissions from vents in the sea bed and volcanos and changes in the biosphere are, I suspect, poorly understood but probably massive compared to the efforts of mankind.
I suspect that the long term increase in atmospheric concentration that we see today is primarily due to the planet warming up from the last ice age and the reducing solubility of CO2 in the warming oceans.
Surely more Co2 going in to the oceans can only be a good thing for all the living animals that relies on it from plankton upwards?
Secondly, IF more Co2 is going in to the oceans then wouldn't research in to plankton growth be a good indicator that this is actually happening?
Sorry, I know Im a complete noob BUT still, it would be nice to know answers to the above (if for no other reason than to allow me to move on with my life:)
Regards
Mailman
The odd thing about the so-called 'missing sink' is that a lot of people have discovered many land-based carbon sinks that had previously not been considered in the first IPCC report yet the IPCC just continues to ignore these discoveries just as they continue to ignore the data from plant stomata written up by several eminent academics in eminent journals that prove the pre-industrial value of 280ppm (obtained entirely by post-hoc rejection of data that didn't conform by a process condemned by Slocum) is nonsense and that the ice-core interpretations suffer from far too little appreciation of natural processes that flatten the peaks.
I wasn't aware though that the IPCC had abandoned their previous deeply flawed carbon budget calc though. As noted above, the error bars dwarf any signal, many assumptions about sinks are founded on sand and the institutionalised bias in using CO2 versus T data from Antarctica (currently cooling and long accepted as having its own unique climate) then extrapolating it over the entire Earth while just ignoring every other dataset from everywhere else on the planet that tells a different story has always been quite appalling 'science'. Notwithstanding all that, these C12/C13/C14 studies also suffer from huge error bars that only the innumerate fail to think is important.
Furthermore I don't believe that squiggle in the Mauna Loa results could have been achieved without significant post-processing: Real background CO2 data varies by 10's of ppm hourly and is incredibly messy. An engineering approach would have been to keep all data then just draw a line at the 5% percentile of the normal distribution but the Keeling procedure is to reject 80% of the data and then stick it in a filter so we can see the (possibly fake) squiggly line of the 'breathing' planet first postulated by Keeling himself: How handy it is to be able to manipulate the data that proves your own hypothesis!
James G
A few links would be helpful!
Alas despite my scepticism of all things to do with CO2 budgets I don't believe it is helpful to discuss whether the CO2 from mankind is really the reason for the increase. This is seemingly beyond the pale even for those true believers who would concede that natural variation has been underestimated in the past. My main scepticism is that CO2 is demonstrably not a driver of climate anyway regardless of where it comes from because we already accidentally conducted a planet-wide experiment based on the alarmists accepted belief that man has increased CO2 from 300 to 400ppm and yet the rate of change of temperature reduced as the rate of change of CO2 increased. That is proof positive that CO2 is not a driver of temperature. At most it can only be a very weak heating feedback, even without considering that the alarmists never bothered to postulate a cooling mechanism for CO2 beyond the notion that massive carbon sinks must just appear out of nowhere by magic.
' human-induced perturbation is reduced as the CO2 spreads through the deep ocean,'
and the proof for this statement is what ?
Like with the 'hidden' heat, the very best thing about the deep ocean is its 'deep and big' therefore much can be claimed to be hiding in it , lost cities , UFO's , C02 safe in the knowledge that others cannot just turn around and say we looked and its not there.
Now in normal science its up to the people making the claim to prove it , and with deep oceans they cannot as they simply don't have the evidenced worth a dam . But in climate 'science' all you need is models and enough faith for your claims to be 'true '
James Hansen believes the sinks are soaking up more than expected, which for him is good news *because* it keeps his dreams of radical carbon cuts alive.
Another problem with the C isotope ration is the fact that man's emissions are in the area of 5% of the total and tiny changes in the ratio of the other 95% will swamp any changes due to man.
Now add in the possibility of yet unknown CO2 emission sources (under sea volcanoes?).
Thanks
JK
The Δ14C from the bomb-spike is now approaching pre-war levels. If the usual IPCC argument is correct then it should shortly (about now, in fact) fall below pre-war levels. If humans are annually adding "cold" carbon to the atmosphere at circa 3% per annum then the 14C should annually be diluted by approx 1/(1.00 +0.03) as the 'natural' chemical disappearance of Δ14C should now be fairly close to the single exponential decay approaching zero.
The most recent high quality Δ14C data from the Jungfraujoch visually does not look to me like it is falling at the ~3%/year dilution rate, despite the authors indirect comments that it is. Look at Figure 1b in the Tellus paper by Levin. (the y-axis scale appears marked strangely as percent, not per-mill which it is in the data table). This suggests that CO2 is not a "well mixed" gas, and that a significant fraction of fossil fuel emissions are getting as far as the bulk atmosphere. There are further conclusions that might be drawn. (At some point I may get round to fitting the data to the exponential decay plus a dilution coefficient.)
I'm going to watch very closely for the next data update.
Paul Siple I've met,
Well dressed for the occasion;
Warm reception.
============
I should have written "a significant fraction of fossil fuel emissions are NOT getting as far as the bulk atmosphere."
It was inevitable that the biome would embellish current and recruit new negative feedbacks with the increase in atmospheric and oceanic CO2. Those plants were starved for CO2.
======================================
"Commenter sounding convincing, but actually talking BS" is a common blog phenomenon.
People often spend a lot of time on the blogs and then start to feel that that they have great understanding. They then go on to make arguments which sound logical and scientific and are made with a great deal of certainty, so that they sound very convincing to many readers. It is only after a while that others expose the flaws. Trouble is that while skeptics do call each other out, warmists don't'. And when skeptics callout a warmist, the warmist and friends often stubbornly stick to their argument shouting " you're only disagreeing with us cos you are denier"
- After a while you'll see the same genius commenting in another thread again with the same false overconfidence. How much time would be saved if people qualified their certainty ?
It has always struck me that this argument about anthropogenic CO2 having a long residence time is entirely circular. Mike MacCracken's post of January 7th acknoweldges that transfers into the deep ocean, whether by humans or otherwise, and transfers out are unknown quantities (and act indepently of one another). So although we know that historically they have been in approximate balance, there is considerable variation which exceeds the anthropogenic contribution.
So if we know that there isn't a straight one to one relationship between a given quantity of anthropogenic CO2 going into the ocean and CO2 coming out, how do we work out how big the human contribution is, ie how long the human pertubation lasts? Well, we just assume that the observed increase in cumulative CO2 must be all be anthropogenic and work back from that.
Then in circular fashion this is taken as proof that the cumulative CO2 is anthropogenic in origin, which is what we've just assumed.
Whilst plants certainly preferentially use C12, because it is lighter I suppose, I do not think that the carbonate shell claim is correct.
Yes, Chris, the ocean itself doesn't even know what it is doing, yet.
============
In the engineering disciplines of Process Control and Reactor Design, the term Residence Time or 'time constant' for a well mixed 'container' has the following form:
Residence Time = (Total Amount of container or system)/ (Exchange amount into or out of system per time)
in consistent units this is
Residence Time = (Total Amount)/(Amount/time) which has units (1/(1/time) which becomes units of Time, hence the name.
Note that on published atmospheric Carbon cycle figures, the annual Exchanges are roughly 20% of the total atmospheric Amount. Residence Time should be about (1/0.2) or 5 years.
For a well mixed, first order system there are equations which describe the system changes over time in Response to changes in the Exchange Amount. In other words, the Response can be estimated from knowing the nature of the system and the Residence Time of the system.
In the comment from MacCracken above, his description (a) is an estimation of the RESIDENCE TIME for the atmosphere, and his description (b) is NOT residence time but the RESPONSE over time to changes in the Exchange.
Why is that distinction important? Well for a step change increase, the 'rule of thumb' is that it takes about 3 to 4 Residence Times for that increase to reach the new level. If you use a Residence Time of 5 to 10 Years, that becomes 15 to 40 years Response. However, if a 30 or 40 year Response is incorrectly used as the Residence Time, and then multiplied by the factor of 3 or 4, now you are up in the century range as we see in some of the more alarming descriptions.
Realistically, the atmosphere has examples of different system changes happening concurrently such as a Step Change (annual increase), a short term Pulse injection (C14 bomb tests), sinusoidal inputs (annual plant growth and decay), and whatever the oceans are doing. The overall Response would then need to account for all of that and further that these changes vary year to year.
" It is known that plants have a slight preference for the C12 isotope of carbon and animals making shells have a slight preference for C13.
What explains the preference for one isotope over another? Presumably it is to do with reaction rates differing. (Or other physical effects?)
If so, I can see why things involving more mobile molecules containing C12 would have a higher rate of uptake than less mobile molecules containing C13. But why should shells go for C13 in preference to C12 in that case?
Anyone...anyone...surely an increase in plankton would be an indicated that the oceans are taking up more Co2 (seeing as they feed on Co2 and eventually return it to the ocean floors as part of the Co2 life cycle)???
So does anyone know IF any studies in to this have been undertaken?
Regards
Mailman
This is a clarification for JamesG. Perhaps I should have been more explicit. If the increase in atmospheric carbon dioxide is natural and anthropogenic emissions don't matter then a lot follows - Irrespective as to whether or not carbon dioxide is an important climate driver the policy reactions of carbon footprint reduction, movement to expensive renewables and the whole mitigation exercise are pointless. It is therefore rather important if my alternate hypothesis is anywhere near correct. As it happens James, study of proxy data over recent times (the last 600 million years) leads me to think it more likely that changes in cloud cover resulting from various mechanisms are more likely to be considerable more important than our old friend carbon dioxide. It seems top me that in all but collisions with other bodies carbon dioxide reacts to temperature changes rather than causing them.
Jungfraujoch is IMO poor data for this kind of analysis.
I looked into the bomb spike matter starting from scratch. There seem to be two datasets of note: north Norway and New Zealand. Data is patchy and not very good. Initial data processing was done as was practical at the time but when this became increasingly inadequate the gas collection and count time were changed (data gap circa 1980). Today other methods have been used but I don't know what is currently used with the only long dataset, nz.
Of course... information is omitted so I have been unable to tie the results to covert preprocessing and gain a full picture. This is the usual story in science. For example there is silence on how and what has been done to compensate for whatever the overall atmospheric CO2 prevalence has done over time. Maybe it doesn't matter but I see no straight discussion.
The fit to exponential is excellent right through to a few years ago, last data I have.
Put one way this is a single pole low pass filter with a cutoff at ~105 years. There is no sign of secondary effects.
A novelty occurred to me Given the phase variation during cutoff this will time skew faster 14C data which is of interest with reconstruction of solar activity / terrestrial linkage. To make life extra fun the data is irregularly sampled hugely complicating any complementary compensation. I have cobbbled up a high pass which accepts irregular sampling and taken a stab at what 14C ought to look like. How exactly this links with the not so simple terrestrial magnetic fields and absorption by growing things is unclear. Or with neutron monitoring which is another can of worms.
And there things stopped. The data is too poor to do much more. I lost interest. We still don't know lots.
On attempting a discussion the usual suspects turn up, trying to turn things back to what they understand. (and I don't)
Henry's law, yes. Attempts are then made to destroy the Henry / CO2 connection within the ocean. This is reminiscent of ice core wars, oh and I have some revelations about ice cores to write up. Oh what a mess.
Don't pay too much attention to the Mauna Loa CO2 dataset for reasons I have not yet revealed. Took me years of work to crack what was going on. Lets not interrupt a mistake.
Funny thing... during the 1970s I was handling the innards of IR gas analysers and the problem of reference.
Lets see what I have, on my own blog this
https://daedalearth.wordpress.com/2013/09/25/strong-evidence-for-linear-law-removal-of-atmospheric-carbon-dioxide/
First plot uses Norway data which can see the Russian tests, second plot the longer New Zealand data which could see the Pacific tests. A graphical derivation of the data held so critical by some is shown, about 15 years. I wrote "Roughly, northern ~15 years, southern ~17 years, near enough the same."
Some more and discussions took place at Tallblokes.
I do my own thing and make mistakes.
About all I can say is that at least one thing is radically not as claimed.
The work of Murry Salby shows that the atmospheric CO2 content over time does not correlate to human emissions and are almost entirely controlled by temperature and soil moisture. If this is true then human emissions have almost no effect on temperature via the greenhouse effect theory. His work has been verified but not yet published. I'm afraid his work has been destroyed by the forces that got him fired. I know it sounds like a conspiracy but I have not been able to find any update on his progress or his work for a year or so.
Regarding residency time, they're correct that the 'molecular' residency time (the five years one) is different to the 'perturbation' residency time (centuries to millenia, maybe).
Start with 900 blue marbles in a bag. Take 500 out at random, and put 500 new blue marbles in. Repeat. The number of marbles remains constant at 900.
Now add 100 red marbles to the bag, making 1000 in total. At the next step, 500 marbles will be removed at random, about 50 of which will be red. Then 500 blue ones will be added. The total will still be 1000. Remove another 500, of which around 25 will be red. Add 500 blue ones. There are still 1000 marbles. After the twentieth repetition it's very unlikely that there will be any red marbles left, but the total is still 1000, ten more than you started with. The excess never diminishes - it's time constant is infinite.
Now suppose that instead of removing 500 marbles we remove 501 each time until the total returns to 900. Remove 501, add 500, remove 501, add 500, etc. After 10 turns, the total will have dropped from 1000 to 990. It will take about 100 repetitions to return to where we started. But it will still only take about six to ten turns to remove all the red marbles, halving the number on each turn.
The residency time for an individual marble is only about 7 turns. But the residency time for the 100 marble excess is about 100 turns. The two numbers are different. Although none of the red marbles remain, the excess that resulted from adding them persists for a long time.
--
Regarding the isotope argument for showing the source of the rise being anthropogenic, the usual IPCC-type argument is incorrect. The presence of fossil-fuel 13C only shows that carbon from fossil fuel is going in, it doesn't show that it is the cause of the rise.
Consider a hypothetical system with strong feedbacks that returns CO2 to a set level in about 20 years - set, for example, by the balance of plankton in the sea. Imagine we add a large dollop of CO2. In about 20 years time, it will disappear and we'll be back to where we were.
Now imagine that in addition we set up a world-wide shipping network that carries plankton from ocean to ocean in their ballast - invasive species galore. Imagine this resets the set level for CO2 about 10% higher. We add our big dollop of fossil CO2, and find that it declines much more slowly afterwards, and never does return to where it started. Twenty years later it's still got another 10% to go. Because we added fossil CO2, the isotope ratio will show its presence in the atmosphere, but after 20 years the excess is entirely due to the plankton and nothing at all to do with the fossil fuels.
The question is, how do you distinguish this sort of hypothetical scenario from the usual no-feedback 'box model' hypothesis? You hit the brakes and the car slowed down, and you initially concluded that the brakes caused that - but it later transpires that the brakes don't work, and the slowdown was actually because you ran out of fuel. How could you tell?
What controls the *dynamics* of the carbon cycle can't be determined from things like the isotope ratio. You need to understand and measure accurately all the potential flows on a global scale. So far as I know, we can't do that at all accurately.
--
Regarding Salby, the effect he identified was already known, and is a consequence of feedback. CO2 affects temperature and temperature affects CO2 - the arrow points in both directions. The long term rise in temperature has added about 10% to the CO2 rise - not enough to explain it. However, short-term changes in temperature are a lot bigger, and lead to bigger short-term variations in CO2, which is what Salby is picking up. In the short term, weather dominates CO2 and temperature leads. In the long term the situation is much less clear.
In any case, what Salby found isn't proof.
Yes DMA. I regard Murry's work as independent agreement with the overall postulate. I was tempted to reference it in my post but rather hoped someone else would mention it as you have done. For a simple summary of Salby's work go to:
http://www.brugesgroup.com/ and click on Climate What we know and What we don't.
Losing your marbles?
Anyhow, there are three fast reservoirs, the atmosphere, the biosphere and the upper oceans. Dump a load of CO2 into any of these, and it will rapidly equilibrate between the three, over a period of ~10 years (Eli is not going to quibble about the difference between 5 and 15 or even 20.) It then takes hundreds or years or more for the fast systems to equilibrate with the lower ocean (which is a much larger reservoir than the other three put together. The flow of carbon out of the deep ocean to the litosphere takes even much longer.
So (as a rough model) consider that you start with 100 marbles in the three fast reservoirs and you put an extra 100 into the atmosphere. Then within about 10 years you will have 133 in each of the three fast reservoirs. That only adds up to 199 you say, where are you hiding the marble, Oh yeah that went into the deep ocean.
Tim, its precision seems very good, and has more recent data than that from NZ.
Those are both very important for the point I was making. The early parts of the curve are less important to my argument here because a) other effects could potentially be invoked, and b) at the earlier times the rate of dilution with cold carbon was small relative to the chemical exchange equilibration.
I am not disputing the simple exponential fit stretching back to the bomb-spike (though others may do). I am stating that it has now become close enough to zero such that the cold-dilution with fossil-fuel CO2 should now be dominant (as Levin states), according to the IPCC. But the numbers are now testing one of the IPCC stated assumptions, and it's not looking very good for them. If/when the Δ14CO2 breaks below the pre-war level, then one of the ambiguities is eliminated. Recent, high precision data will bring the moment of truth sooner (within about 3 years, IMO). Less precise data will bring it later. But it will arrive at some point. They can run, but they can't hide.
Since the commjents are degrading to nit picking that convinces no one, I shall add my own bothersome nit that is off topic, referencing the language rather than the substance of the post.
"… Also recall than[sic] man's CO2 is different …" has an improper use of the word, 'than'. For some reason, of late, the confusion of the meanings of that, then and than seems to be on the increase. I often see the misuse of 'that' in a similar manner, "John is older that Jane." The word 'then' gets thrown in in any old place.
From Webster,
Than \Than\ ([th][a^]n), conj.
A particle expressing comparison, used after certain
adjectives and adverbs which express comparison or diversity,
as more, better, other, otherwise, and the like. It is
usually followed by the object compared in the nominative
case. Sometimes, however, the object compared is placed in
the objective case, and than is then considered by some
grammarians as a preposition. Sometimes the object is
expressed in a sentence, usually introduced by that; as, I
would rather suffer than that you should want.
For comparison, the word 'that' is used as a demonstrative pronoun or article. For example, the book, that book, these books &c. As my German nanny (lo, these three score years ago) would practice, "this, that, these, those and the others."
Now, we return to our regularly scheduled program.
Thank you.
Gary Turner, I think your nit picking doesn't make sufficient allowance for errors potentially introduced by typing errors or 'correction' by a spell-checker. The grammar in the "commjents" you object to could just be an artifact of a mindless automaton.
You're welcome.
"Anyhow, there are three fast reservoirs, the atmosphere, the biosphere and the upper oceans. Dump a load of CO2 into any of these, and it will rapidly equilibrate between the three, over a period of ~10 years (Eli is not going to quibble about the difference between 5 and 15 or even 20.)"
There's also the geological reservoir - carbonate rocks. There's the fossil fuel reservoir - more fossil fuels are being slowly created. And for the really long-term the slow evaporation of gases into outer space. And of course you can subdivide your four reservoirs into different elements with potentially different behaviour. Do different ocean basins, deep/shallow water, or warm/cold water all work the same way? Do different species within the biosphere all have the same time constants?
But that doesn't address the point that there may be other inputs affecting the equilibrium positions, and that the potential causes cannot be distinguished from the output. If CO2 rose because one of the unobserved biosphere reservoirs got wiped out by disease, how would you distinguish the resulting rise of the CO2 from other potential causes using your 4-box model?
I'm not saying I think that's what happened - I think the anthropogenic hypothesis is the best available one myself. There's no evidence to support any of the others. But there's a gap in the logic of the argument when it comes to claiming other hypotheses have been eliminated. 'Proof' is a much stronger claim than 'best supported'.
Another thing is that the residency time (the overflowing bathtub analogy) argument actually contradicts the notion that the 2% manmade contribution is detectable by isotope ratios since the overburden would be still entirely natural. You can argue that the excess CO2 is demonstrably manmade or you can argue that there is an excess due to the overflowing bathtub effect but you cannot sensibly argue both at the same time. It's both innumerate and illogical!
RE Carbon sinks.
I recall seeing this NOVA program last year - http://www.pbs.org/wgbh/nova/earth/earth-from-space.html
Apparently, scientists, until recently, were not aware of the extent of plankton blooms in the open ocean. This strikes me as a possibly significant sink not being accounted for. Rather than it taking millenium for atmosphereic carbon to be entrained as ocean sediment, this looks like it happening in a period of months.
"The fit to exponential is excellent right through to a few years ago, last data I have." --Tim Channon
From about 1995 to present, the Mauna Loa data is linear, except for the seasonal wiggles. .
"There's also the geological reservoir "
REALLY SLOW
There's the fossil fuel reservoir
Being steadily depleted. Building back is even slower
In short no.
"REALLY SLOW"
Yep. So's the deep ocean.
"In short no."
No... what? No, they're not carbon reservoirs? No, they don't have long time constants? No, they don't extend the time taken to reach equilibrium? No, your 4-box model is a complete description of the system and there's no possibility of any other effects being important? Or did you think I was saying something else?
Yes the three fast reservoirs and the slower ones are all reservoirs, but because the rates of exchange are so radically different the three fast reservoirs equilibrate amongst themselves essentially immediately (within a few years) while the leakage rate out of the fast ones takes centuries.
So for the purpose of figuring out what is happening in years if not decades you can pretty much ignore the slower processes.
The converse of this is that you can usefully lump the three fast reservoirs together with a single rate for moving carbon into and out of the slow reservoirs for a decent back of the envelope model
Of course, you could always try to find a catalyst to speed up the sequestering of CO2
This is all elementary kinetics so don;t try the hot and bothered..
@Michael Hart:
I had a quick look at the D14C data from Jungfraujoch and find the half-life to be 12.2 years for single exponential decay. If a background level is assumed then it drops to 11.4years. Both have adjusted R^2 of 0.9780.
Nullius in Verba @ Jan 15, 2015 at 8:04 PM
"Regarding Salby, the effect he identified was already known..."
Not so much. It was known that temperatures affect the rate of change of CO2, but it was not appreciated that the temperature to rate of change relationship accounts for essentially all of the CO2 observations, and that significant human attribution is thereby ruled out.
It is quite apparent that CO2 is a temperature dependent process which obeys a differential relationship of the form
dCO2/dt = k*(T - Teq)
where k is a coupling factor and Teq an equilibrium temperature at which atmospheric concentration would remain unchanged. It has obeyed this relationship for essentially constant values of k and Teq since at least 1958, when measurements at MLO commenced.
We do not even need proxy measurements from before this modern era to tell us what has been going on with CO2 over this, the era of the greatest portion of the observed rise from putative pre-industrial levels. This set of data tells the whole story for all practical purposes.
The slope of the plot above is fully explained by the temperature relationship. Human emissions also have a slope but, since it is already accounted for by the temperature relationship, they cannot be having a significant impact.
A dynamic such as above could come about if there were upwelling of CO2 enriched waters from the depths. Such upwelling would inexorably outgas to produce a continually advancing atmospheric equilibrium. An overall system response would be similar to the following:
dCO2/dt = (CO2 - CO2eq)/tau + A
dCO2eq/dt = k*(T - Teq)
These equations describe a system which regulates atmospheric CO2 to the level CO2eq set by the ocean interface. For a short time constant tau, the contribution from anthropogenic inputs A would be on the order of A*tau, i.e., very much smaller than the sum total of A. CO2 would track CO2eq, and the overall result would be consistent with the observations.
For the actual system, there might be a series of time constants associated with different sink diffusion rates, but the outcome is effectively the same. There is little doubt that human inputs are insignificant in the overall process, as human inputs are not modulated by temperature.
This is a very ordinary feedback process. Indeed, when someone says "But, atmospheric concentration tracks human emissions very well," the answer is, "No, they do not." A comparison of human emissions with atmospheric concentration shows that emissions are still accelerating (slope of the lines in these curves), while the rate of change of atmospheric concentration has come to a standstill in the years since the plateau in temperatures.
On residence time versus persistence time - It is said that the C14 decay is representative of the rate of dilution into competing reservoirs, and not indicative of the overall persistence time. True, it is not necessarily so, but conversely, it is not prohibited from being so.
If the sinks are very active, and indications are that they are (see above post), then the persistence time and residence time necessarily converge with one another.
I was rather hoping that Mike McCracken would continue with his conversation on the Bishop Hill blog. However, he continued on ClimateSceptics with the following:
Lots of detail in the response, but two clarifications:
1. In saying that we tend to agree on the lifetime of a particular CO2 molecule in the atmosphere being about 5 years before being taken up by ocean or biosphere (roughly balanced by one being emitted back to the atmosphere), you say that:
“Of course, this contrasts somewhat with IPPC’s assumption for models of circa 100 years but without going into the matter in more detail this is probably because IPCC assume that the natural level to which CO2 needs to fall is its stated and so-called pre-industrial level of 280 ppm.”
The comparison being made is apples and oranges. When IPCC gives a number of order 100 years (in lieu of giving four different exponentials), they are talking about the effective lifetime (well, the near-term exponential decay time) of the human-induced perturbation to the CO2 concentration—not the lifetime of a particular CO2 molecule. This is a critical difference.
2. On this issue of there being a lot of different estimates of the atmospheric lifetime of a particular CO2 molecule, there was indeed a range in early papers as the rate of ocean uptake and and release was not really worked out until the 1950s and 1960s when, for example, Suess and Revelle got clear data on the separation of the upper ocean from the deep ocean. A lot has been learned as the result of tracking radionuclides generated by testing of nuclear weapons during the mid-20th century and also of uptake of various other tracers (like CFCs, etc.)--some of which are involved with the terrestrial biosphere and some not, and some of which have atmospheric chemistry or ocean sinks and some not, etc. So, yes, refinement of this has been going on over time, and there are various reasons for minor differences, but it is quite clear that a good rough estimate of the lifetime of a particular CO2 molecule in the atmosphere is roughly 5 years, and that, quite separately, transport and spreading of the excess CO2 concentration into and through the deep ocean takes of order 1000 years.
I responded with:
@MikeMcC: If only the argument were about residence times then you would have a point. However, my argument is that if the natural emission of CO2 is increasing and predominates then anthropogenic emissions do not matter. It follows that in such circumstances policies aimed at reducing the anthropogenic component by increasing the use of very expensive renewable energy technologies is both pointless and wasteful. The corollary is that we had better do whatever is necessary to determine the mechanism in charge and such determination should not be as per IPCC i.e. an assumption.
The reply:
Given the relative stability of the CO2 concentration during the Holocene prior to the start of industrial emissions, could you summarize the reasons for thinking that natural processes would be the cause of the CO2 concentration going so rapidly from about 280 to now 400 ppm? And especially since there is no indication of the CO2 concentration being this high in millions of years.
And my response:
@MikeMcC re: your post on Sunday 18 January. Your first sentence: yes I could but I think for the time being I shall leave it to others. I would dispute your second sentence and suggest you read Zbigniew Jaworowski's papers. Sadly I cannot ask him to join in the conversation as he died recently. You may also like to comment on the Murry Salby stuff that I chose not to include knowing that others would pick it up notably Bart (see http://bishophill.squarespace.com/blog/2015/1/15/carbon-conversation.html)
I think that I will leave things parked as I do not expect that Mike will appear here – but one never knows? Best to all Regards Peter
"Given the relative stability of the CO2 concentration during the Holocene prior to the start of industrial emissions, could you summarize the reasons for thinking that natural processes would be the cause of the CO2 concentration going so rapidly from about 280 to now 400 ppm?"
This is a subtle yet powerful reason that either
A) Earthly CO2 is tightly regulated, with rapid attenuation of small disturbances so that human inputs should have little effect, or
B) the imputed CO2 measurements from the ice core record are unreliable in the manner they have been interpreted (as Salby maintains)
Very low bandwidth is inconsistent with tight regulation. Very low bandwidth feedback systems do not maintain tight regulation within a narrow band - they wander.
Each random variation in the input accumulates over time such that, in the interval of time much less than the dominant time constant, the 1-sigma bound grows with the square root of time. See Brownian Motion and Wiener Process. This is not what we see in the ice core record, leading to the above conclusions.